The compound hcooch ch2 h2o, a fascinating blend of organic and inorganic chemistry, offers an intriguing glimpse into the complexities of molecular interactions. As we delve into this unique structure, we uncover its significance in various scientific fields. From understanding its molecular components to exploring its applications in industry and biology, this blog post aims to illuminate the intricate dance between these two branches of chemistry. Join us on this journey as we dissect the chemical dynamics and real-world implications surrounding hcooch ch2 h2o.
The Interplay of Organic and Inorganic Chemistry”
The exploration of hcooch ch2 h2o reveals the fascinating interplay between organic and inorganic chemistry. This compound serves as a bridge, showcasing how molecular structures can influence reactions and interactions within both realms.
By analyzing its components, chemical equations, and mechanisms, we gain insights into its industrial applications and biological relevance. The significance extends beyond mere theory; it encompasses environmental considerations and educational importance in understanding fundamental chemical principles.
Introduction to hcooch ch2 h2o
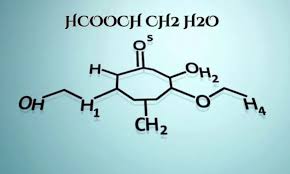
HCOOCH2H2O represents a unique compound that bridges organic and inorganic chemistry. This simple molecular structure consists of formate (HCOO) attached to a methylene group (CH2), with water as an integral component, highlighting its importance in various chemical processes.
Understanding this compound is crucial for researchers exploring its multifaceted roles in biochemical applications and industrial production. Its properties exemplify the intricate relationships between different branches of chemistry, making it a subject worthy of detailed investigation.
Understanding the Molecular Components
The molecular structure of hcooch ch2 h2o highlights its significance in both organic and inorganic chemistry. Comprising carbon, hydrogen, and oxygen atoms, this compound features an ester functional group that defines its chemical behavior.
The presence of the hydroxyl group (–OH) allows for unique interactions with water molecules. This interplay facilitates solubility and reactivity within various environments, making it critical for applications across multiple scientific disciplines. Understanding these components is essential for further exploration of their potential uses.
Chemical Equation and its Mechanism
The chemical equation representing the interaction of hcooch ch2 h2o showcases an ester hydrolysis reaction. In this process, the ester bonds are cleaved by water molecules, resulting in the formation of carboxylic acids and alcohols.
This mechanism involves nucleophilic attack where water acts as a nucleophile. The hydroxyl group from water targets the carbon atom within the carbonyl group of the ester. This results in intermediate formations that ultimately yield distinct products through proton transfers and bond reorganizations within molecular structures.
Applications and Industrial Production
Hcooch ch2 h2o plays a vital role in various industrial applications, particularly in the production of esters and solvents. Its unique molecular structure allows it to act as an efficient reagent in organic synthesis, making it indispensable for pharmaceuticals, agrochemicals, and fragrances.
Moreover, this compound is utilized in polymer chemistry for creating biodegradable materials. The growing interest in sustainable practices drives its demand across industries as manufacturers seek environmentally friendly alternatives to traditional chemicals.
Biological Relevance and Enzymatic Hydrolysis
The biological relevance of hcooch ch2 h2o is significant, particularly in metabolic pathways involving ester compounds. These molecules serve as essential intermediates in various biochemical reactions, facilitating the synthesis and breakdown of vital biomolecules.
Enzymatic hydrolysis plays a crucial role in this context. Enzymes such as lipases and esterases catalyze the cleavage of esters, aiding in nutrient absorption and energy production. This process underscores how organic and inorganic chemistry intertwine within living systems to maintain cellular homeostasis.
Catalyzed Mechanisms and Educational Importance
Catalyzed mechanisms, particularly in the context of hcooch ch2 h2o, illustrate essential principles in both organic and inorganic chemistry. By exploring these pathways, students gain insight into reaction kinetics and thermodynamics.
Educationally, such experiments foster critical thinking and problem-solving skills. They provide a practical understanding of how catalysts influence reaction rates while emphasizing the importance of environmental factors. Engaging with these mechanisms strengthens foundational knowledge crucial for advanced chemical studies across various disciplines.
Ester Hydrolysis Experiment Guide
To conduct an ester hydrolysis experiment, prepare a solution of the ester (hcooch ch2 h2o) and add hydrochloric acid as the catalyst. Heat the mixture gently in a water bath for optimal reaction conditions.
Monitor the progress using thin-layer chromatography to determine when hydrolysis reaches completion. Post-reaction, neutralize with sodium bicarbonate before extracting products with an organic solvent. This practical approach offers insights into kinetics and mechanisms inherent in organic reactions while reinforcing laboratory skills essential for chemistry students.
Environmental Considerations and Green Chemistry
The synthesis and application of hcooch ch2 h2o raise important environmental considerations. Traditional methods often involve hazardous solvents and by-products that can harm ecosystems. Emphasizing green chemistry principles helps mitigate these impacts.
Innovative approaches focus on reducing waste, utilizing renewable resources, and ensuring energy efficiency during production processes. Adopting such practices not only aligns with sustainability goals but also enhances the overall safety profile of chemical research in both laboratory settings and industrial applications.
Computational Studies and Molecular Modeling
Computational studies play a vital role in understanding hcooch ch2 h2o at the molecular level. These techniques facilitate the prediction of molecular behavior, providing insights into reaction mechanisms and stability.
Molecular modeling allows researchers to visualize structures and interactions that are often challenging to observe experimentally. By simulating various scenarios, scientists can explore potential pathways for reactions, optimizing conditions for chemical processes involving this compound and enhancing its applications across multiple fields.
Historical Background and Research Development
The exploration of hcooch ch2 h2o has roots in the early studies of organic compounds and their interactions with water. Chemists in the 19th century began investigating esterification and hydrolysis reactions, laying foundational principles for understanding this molecular structure.
Research development accelerated throughout the 20th century with advances in analytical techniques. These methods allowed scientists to probe deeper into molecular behavior, fostering a richer comprehension of both organic and inorganic chemistry’s interconnectedness through compounds like hcooch ch2 h2o.
Chemical Safety and Lab Protocols
Chemical safety is paramount when working with hcooch ch2 h2o and related compounds. Proper lab protocols must be adhered to minimize risks associated with handling organic and inorganic substances. This includes wearing personal protective equipment (PPE) such as gloves, goggles, and lab coats.
Additionally, understanding Material Safety Data Sheets (MSDS) for each chemical involved is crucial. These documents provide essential information on hazards, safe handling practices, and emergency measures in case of accidental exposure or spills.
Conclusion and Significance
The exploration of hcooch ch2 h2o reveals a rich tapestry woven from organic and inorganic chemistry. Understanding its molecular components enhances our grasp of chemical interactions and reactions critical for various applications.
Moreover, the significance extends beyond academia into industrial processes and biological systems. The interplay between these disciplines not only fosters innovation but also addresses pressing environmental concerns through sustainable practices. Continued research in this area holds promise for advancing both scientific knowledge and practical applications in diverse fields.
FAQs and Common Questions
As we delve into the complexities surrounding hcooch ch2 h2o, several questions often arise among students and researchers.
What is the significance of this compound in organic chemistry? Its dual nature connects organic molecules with inorganic properties, making it a vital subject of study.
How does enzymatic hydrolysis work? This process involves breaking down esters like hcooch ch2 h2o through specific enzymes, showcasing its biological importance.
Are there environmental concerns related to its use? Awareness of eco-friendly practices is essential when handling compounds within this category.
For further inquiries or deeper explorations into hcooch ch2 h2o’s multifaceted role in science, engaging with academic literature can provide valuable insights.